ICH E6(R3)에서 새롭게 등장한 Data Flow Diagram, 왜 필요한가?
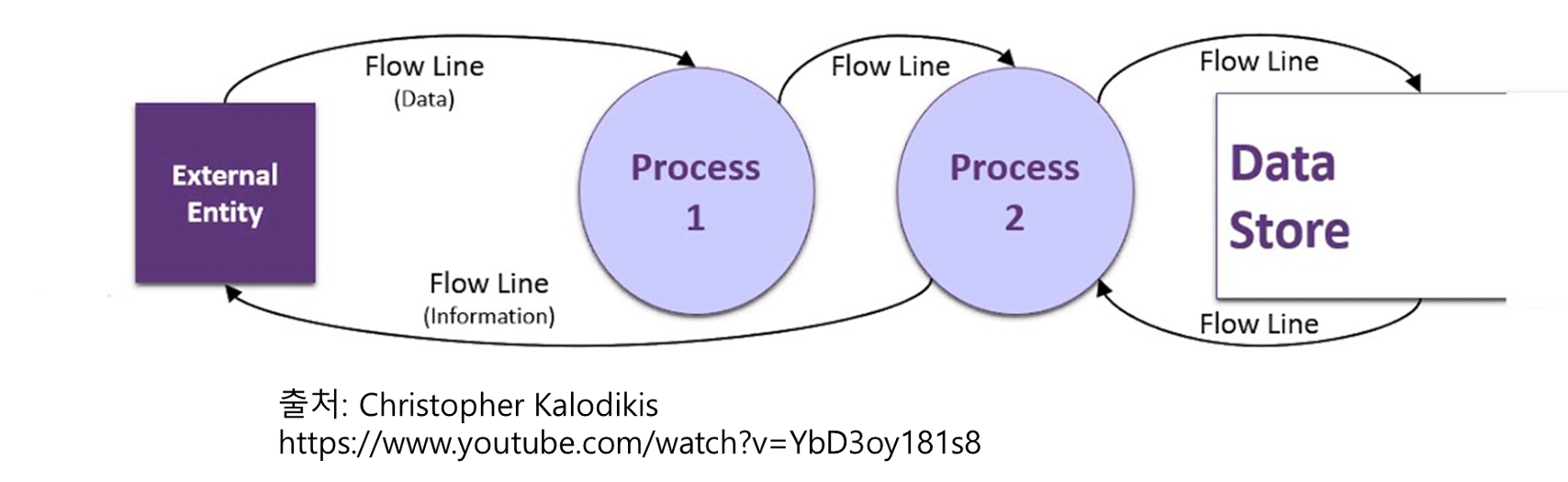

Data flow diagram (DFD)은 1970년대 소프트웨어 엔지니어 Larry Constantine과 Ed Yourdon이 『Structured Design』에서 처음 소개한 기법으로 알려져 있습니다. 이후 Tom DeMarco, Chris Gane, Trish Sarson 등이 표준화된 기호와 표기법을 정립하면서 오늘날까지 널리 쓰이는 데이터 흐름 표현 도구가 되었습니다. DFD의 핵심은 “데이터가 어디에서 생성되어, 어떤 프로세스를 거쳐, 어디에 저장되고, 다시 어디로 흘러가는지”를 직관적인 그림으로 … ICH E6(R3)에서 새롭게 등장한 Data Flow Diagram, 왜 필요한가?






